1. The general view of plastic masterbatch
Plastic masterbatch could be seen as polymers masterbatch. Polymers can be made from many different kinds of ‘mers’ which stands for chemical units. Most chemical units are sourced from oil or other hydrocarbons. Hydrocarbons are just as they appear, made of hydrogen and carbon. So, plastics are made of (mostly) hydrogen and carbon that have been assembled together to form mers (like ethylene or propylene), and then these mers link to form chains and when these chains are long enough to become ‘poly’ usually when at least 100 of the mers have linked together, we get to have a plastic/polymeric material.
 The plastic masterbatch of the thermoplastic family is made up of long-chain molecules comprising mainly carbon and hydrogen called polymers. The word combines “poly” referring to many and “mer” referring to the individual molecular repeat units that are chained together. The mer components of the different types of plastics, the strength of the molecular bonds that hold the mers to each other, and the length of the polymer chains are the primary determiners of the plastic properties. Some plastics alternate more than one type of mer units. Plastics masterbatch of the thermoset family while similar to the ones described above have different linkages between mers including cross-links that give them different properties including in some cases higher temperature capability and inability to melt before decomposing as the temperature increases.
The plastic masterbatch of the thermoplastic family is made up of long-chain molecules comprising mainly carbon and hydrogen called polymers. The word combines “poly” referring to many and “mer” referring to the individual molecular repeat units that are chained together. The mer components of the different types of plastics, the strength of the molecular bonds that hold the mers to each other, and the length of the polymer chains are the primary determiners of the plastic properties. Some plastics alternate more than one type of mer units. Plastics masterbatch of the thermoset family while similar to the ones described above have different linkages between mers including cross-links that give them different properties including in some cases higher temperature capability and inability to melt before decomposing as the temperature increases.
2. Breaking down the plastic masterbatch
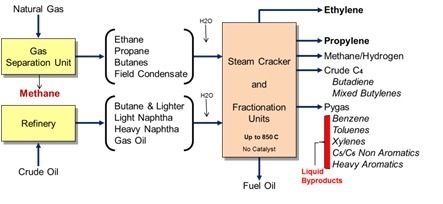
A lot of plastic masterbatches are cracked in crackers! Steam crackers in the main. But not exclusively. There are a whole bunch of thermoplastics that are derived from a raw material called ethylene. And ethylene is produced in a variety of ways, mainly from either oil or gas feedstock. One very popular way is to put the feedstock into a steam cracker and outcomes ethylene, plus some other stuff too. The ethylene is then polymerized into polyethylene or polypropylene mainly but not exclusively. PVC, PS, PET, and butadiene are also made. Here is an excerpt from an article explains that plastic masterbatch is much better: "Ethylene is the starting point for four very mature end products: polyethylene (three types: LDPE, LLDPE, and HDPE), ethylene oxide, ethylene dichloride (the precursor to vinyl chloride monomer), and ethylbenzene (the precursor to styrene). Smaller-volume, more specialized products include linear α-olefins, vinyl acetate monomer, synthetic ethanol, etc." Following is the list of some popular ethylene masterbatch products:
| PVA Poly(vinyl acetate), poly(vinyl alcohol) |
PET Poly(ethylene terephthalate) |
| PVC Poly(vinyl chloride) |
PS Polystyrene |
| LLDPE Linear low-density polyethylene |
PEG Poly(ethylene glycol) |
| LDPE Low-density polyethylene |
HDPE High-density polyethylene |
By far the predominant manufacturing route to ethylene is steam cracking gaseous feedstocks (ethane, propane, or butane) or liquid feedstocks (naphtha or gas oil). At an extremely high temperature of 850 Celsius degree or much higher, a systematic series of mechanized non-catalytic cracking has been run. Ethylene is the intended product; but other valuable building-block molecules, such as propylene, butadiene, and benzene, are co-produced. The yield of each coproduct is mostly a function of the feedstock used. Cracking ethane gives almost no coproducts; but cracking naphtha provides substantial amounts of propylene, butadiene, and benzene. As viewed worldwide, steaming cracking could be seen as one of the most vital resources of butadiene, propylene, and the subordinate leading resource of benzene. The following picture illustrates the schematic of systematic series of mechanized steaming cracking in the most simple way.